Inhaled drug delivery systems face unique stability challenges that need to be managed in order to meet label claim dosages and improve patient compliance. From keeping powder formulations dry within a delivery system, to controlling the build-up of powder in the delivery channel, to maintaining the integrity of APIs within capsule- and reservoir-based DPIs (dry powder inhalers) and pMDIs (pressurized metered-dose inhalers), Aptar CSP Technologies can custom-design a solution to meet your drug product’s stability needs so you can deliver an optimal patient and consumer experience.
Aptar CSP’s proprietary 3-Phase Activ-Polymer™ platform technology can be integrated into virtually any inhaled drug product to adsorb moisture, oxygen or volatile organic compounds, mitigating failure of product integrity and/or stability.
Solutions for inhaled drug devices can be designed in a variety of configurations including:
- Active Molded Components that serve as an integral part of the inhaled drug delivery device
- Secondary Packaging Solutions
- Activ-Blister™ Solutions to protect individual blister cavities enclosing capsules for capsule-based DPIs
3-Phase Activ-Polymer™ Components
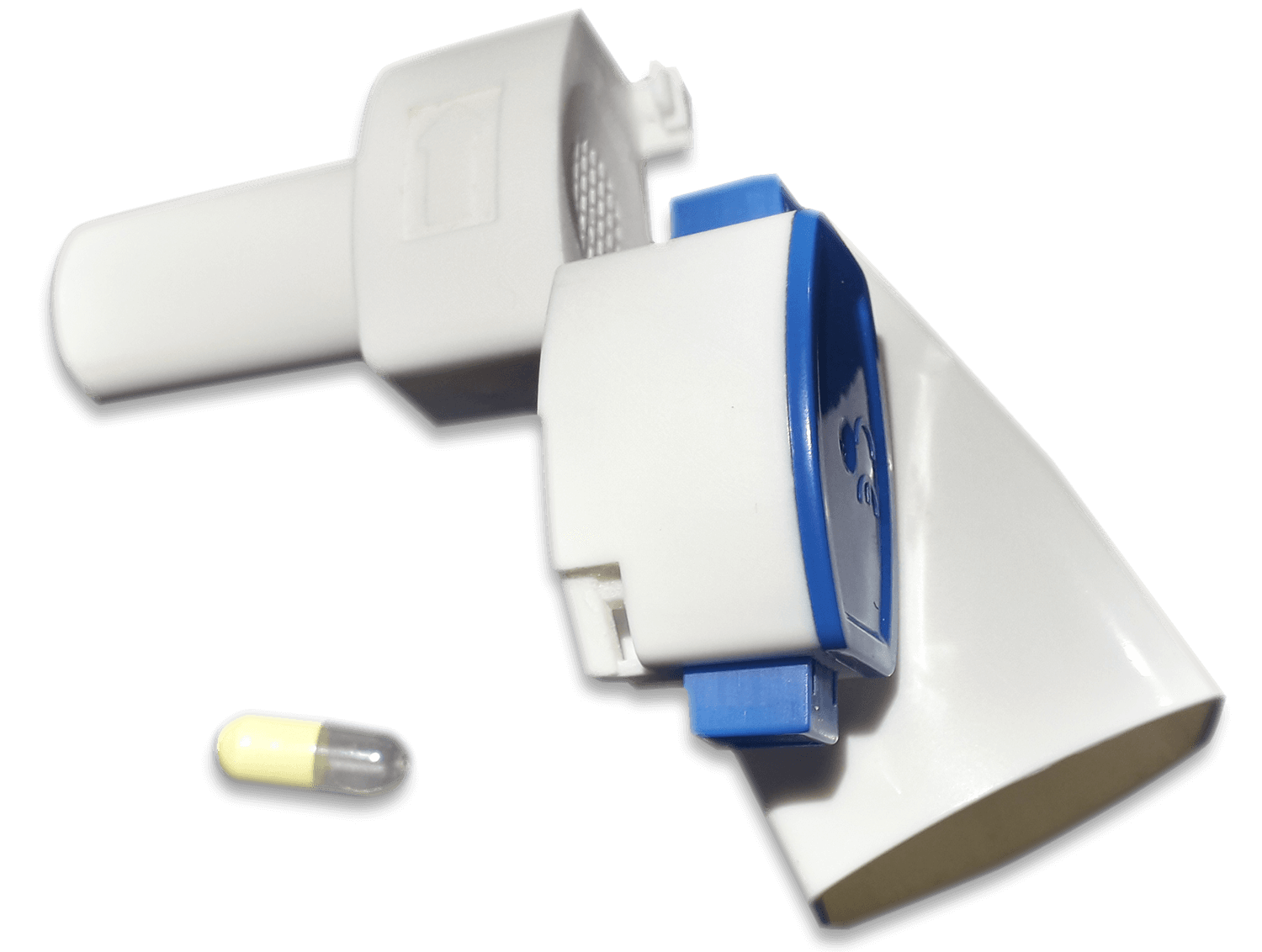
Active molded components can be seamlessly integrated into an inhaled drug delivery device (DPI and pMDIs) by replacing a current plastic part in the device, providing the part’s physical functioning while incorporating the material science performance. As an example, pMDIs face chronic challenges with post-actuation particle build-up at the stem valve, spray nozzle, and mouthpiece of the actuator. Using an integrated active molded component around the canister or within the actuator can prevent these often ignored limitations that cause sub-therapeutic treatment. DPIs offer a different challenge, whereby keeping the powder formulation protected from the outside environment, from bulk formulation, to blister filling, to finished product manufacturing, is critical to maintaining dose integrity. Integrating an active molded component inside a device, particularly reservoir-based DPIs, ensures a drier environment for the device’s delivery pathway, reducing build up and ensuring better dosage delivery for patients.
Activ-Blister™ Solutions for Capsule-Based DPIs
Activ-Blister™ solutions ensure greater efficacy and compliance for capsule-based DPIs. This innovative technology integrates Aptar’s proprietary 3-Phase Activ-Polymer™ film into each individual blister cavity to provide the ideal microclimate to enhance capsule stability within a thermoformed blister package.
Using highly-engineered 3-Phase Activ-Polymer™ technology, Activ-Blister™ can adsorb tailored amounts of water vapor, scavenge oxygen or VOCs, or provide a combination of active protection. The technology can be fitted to accommodate any tablet or capsule size and is securely bound to the lid stock using our proprietary heat staking technology.
Secondary Packaging Solutions
3-Phase Activ-Polymer™ technology can be integrated into secondary packaging for inhaled drug devices to protect the entire device from moisture, oxygen, and reactive impurities and extend shelf life. The packaging can have 3-Phase material inside to provide the protection, or can utilize Activ-Film™ technology that is heat staked to the package’s foil seal.
Why Aptar CSP Technologies?
- Custom-engineered solutions that can be seamlessly integrated into current or new designs
- Active protection without impacting device design
- Heat staking process for film is adhesive free and eliminates residual solvents that can off-gas and interact with drug product
- Reduced package complexity